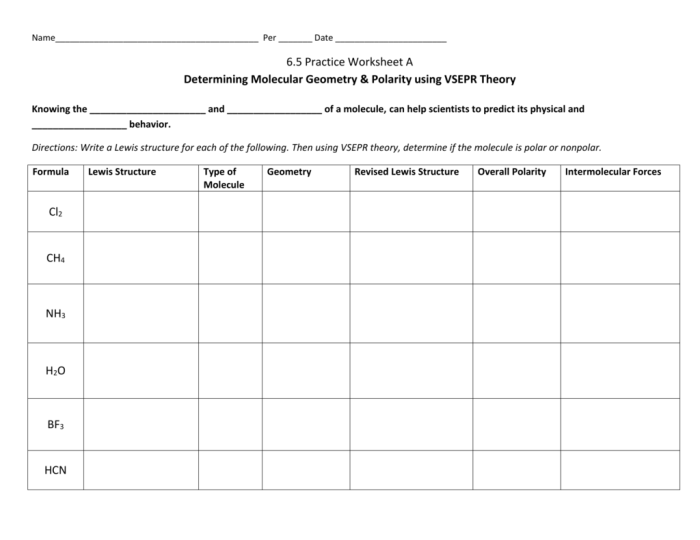
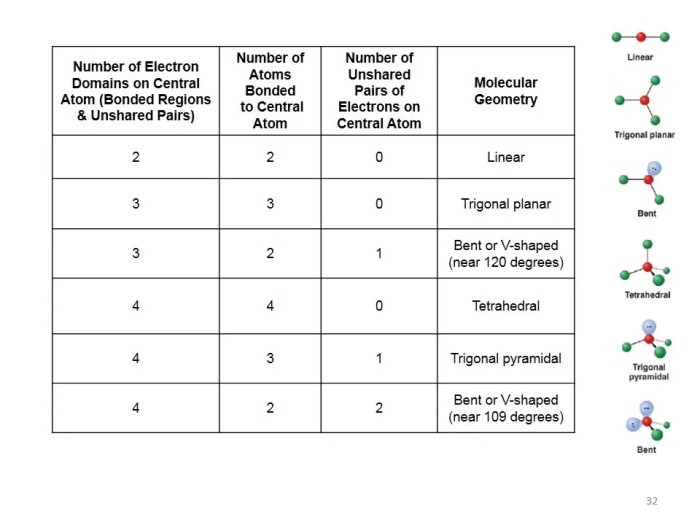
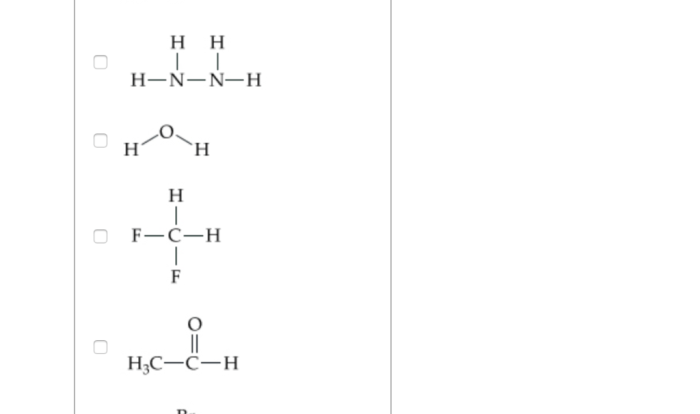
Delving into the realm of molecular shape and polarity, this comprehensive guide serves as an authoritative resource for students and practitioners alike. Our molecular shape and polarity worksheet answers provide a structured approach to understanding these fundamental chemical concepts, empowering you to excel in your academic pursuits and professional endeavors.
This guide encompasses the intricacies of molecular shape and polarity, exploring their interplay and significance in various applications. With a focus on clarity and precision, we present a thorough analysis of molecular properties, providing you with a solid foundation in this essential field.
Molecular Shape and Polarity
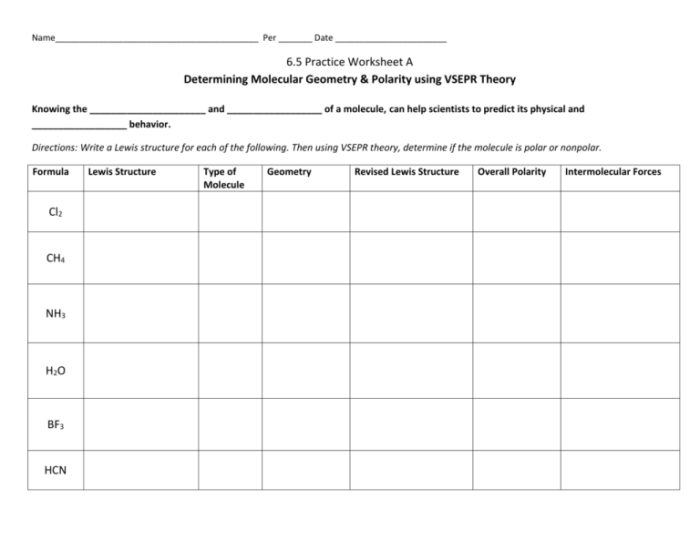
Molecular shape refers to the three-dimensional arrangement of atoms within a molecule. Polarity, on the other hand, refers to the uneven distribution of electrons within a molecule, resulting in a separation of positive and negative charges.
The molecular shape and polarity are closely related. The shape of a molecule influences the polarity of its bonds, and the polarity of the bonds, in turn, affects the overall shape of the molecule.
Worksheet Answers
1. What is the molecular shape of water? Answer: Bent
2. Is carbon dioxide polar or nonpolar? Answer: Nonpolar
3. Explain why ammonia is polar. Answer: Ammonia has a trigonal pyramidal shape with a lone pair of electrons. The lone pair creates an uneven distribution of electrons, resulting in polarity.
Applications of Molecular Shape and Polarity
The understanding of molecular shape and polarity has numerous applications in various fields:
- Drug Design:The shape and polarity of drug molecules can influence their interactions with biological targets, affecting their efficacy and toxicity.
- Material Science:Tailoring the shape and polarity of materials can enhance their properties, such as strength, conductivity, and optical behavior.
- Chemical Separations:Polarity differences can be exploited in techniques like chromatography to separate and purify compounds.
Interactive Table, Molecular shape and polarity worksheet answers
| Molecular Name | Shape | Polarity | Examples |
|---|---|---|---|
| Water | Bent | Polar | H2O |
| Carbon dioxide | Linear | Nonpolar | CO2 |
| Ammonia | Trigonal pyramidal | Polar | NH3 |
Questions Often Asked: Molecular Shape And Polarity Worksheet Answers
What is molecular shape?
Molecular shape refers to the three-dimensional arrangement of atoms within a molecule.
How does molecular shape affect polarity?
Molecular shape influences polarity by determining the distribution of electrons within the molecule.
What are the applications of molecular shape and polarity?
Molecular shape and polarity play crucial roles in fields such as drug design, materials science, and biochemistry.